Abstract
Background
Each year more than 90,000 cases of chronic lymphocytic leukemia (CLL) and non-Hodgkin lymphoma (NHL) are expected in the US. The standard of care is immuno-chemotherapy with alkylating agents in combination with an anti-CD20 monoclonal antibody (mAb). While immuno-chemotherapy regimens are initially effective in inducing responses, most patients inevitably relapse and the same therapies show decreasing efficacy with repeated administration.
In recent years, CD37 has been considered as a target for B-cell malignancies. CD37 is strongly and selectively expressed on the surface of mature B lymphocytes and B-cell malignancies. Therapies targeting CD37 expressing cells may become a useful alternative to CD20 targeting agents. Alpha-emitting radionuclides have demonstrated good potential for cancer targeted therapies because of efficient energy deposition along the short alpha track (50-100 µm). The absorbed energy cause irreparable DNA double-strand breaks and localized cytotoxicity while sparing surrounding healthy tissues. We have developed a targeted alpha therapy (TAT) where the CD37-specific antibody NNV003 is coupled to the alpha-particle-emitting radioisotope 212Pb.
Materials and Methods
Dose-dependent efficacy and tolerability of a single-dose 212Pb-NNV003 treatment was evaluated using escalating activity levels in human disseminated models of Burkitt's lymphoma (Daudi) and CLL (MEC-2). 10 million Daudi cells or 2.5 million MEC-2 cells were intravenously injected in CB17-SCID or R2G2 mice and 212Pb-NNV003 was given two days later. Unspecific, 212Pb-labeled antibody was used as control. A study of the tolerability of 212Pb-NNV003 was performed in R2G2 mice without tumor prior to the efficacy studies.
Results
212Pb-NNV003 displays a favorable toxicity profile in tumor-free mice single intravenous dose injections up to 15 µCi showing 100 % survival 4 weeks post-injection. No acute hematological toxicity was observed and animals who received 5, 10 or 15 μCi doses of 212Pb-NNV003 presented only a slight initial reduction in their platelets (PLT) counts which was fully recovered by 20 days after injection.
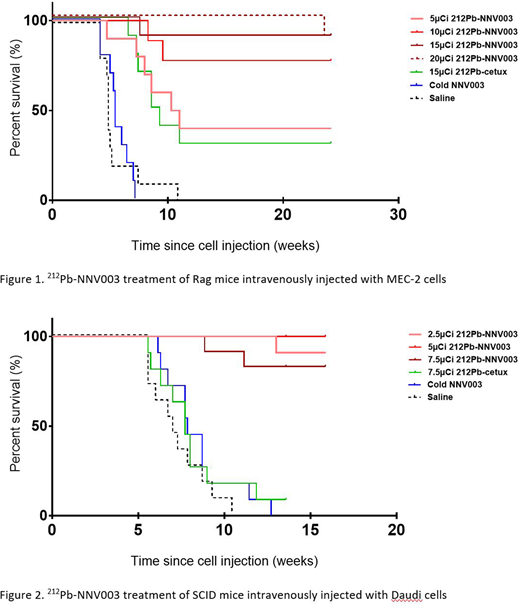
A single intravenous dose of 10, 15 or 20 µCi of 212Pb-NNV003 lead to 70 %, 90 % and 100 % of mice injected with MEC-2 cells being tumor free 20 weeks post cell injection (Figure 1). Control animals that received saline, cold antibody or 212Pb-cetuximab presented a median survival of 4.9, 5.4 and 9.3 weeks, respectively.
A single intravenous dose of 2.5, 5 and 7.5 µCi 212Pb-NNV003 lead to over 80% tumor-free mice injected with Daudi cells 15 weeks post cell injection (Figure 2). Control animals that received saline, cold antibody or 212Pb-cetuximab presented a median survival of 7, 7.8 and 7.7 weeks, respectively.
Conclusion
The results of preclinical studies suggest that TAT using 212Pb-NNV003 is a safe and effective method for the treatment of CD37 positive CLL and NHL.
Saidi:Orano Med: Employment. Maaland:Nordic Nanovector: Employment. Torgue:Orano Med: Employment, Membership on an entity's Board of Directors or advisory committees. Heyerdahl:Nordic Nanovector: Employment, Equity Ownership. Dahle:Nordic Nanovector ASA: Employment, Equity Ownership, Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal